
What the Health
Price: (as of – Details) What the Health is the groundbreaking follow-up film from the creators of the award winning documentary Cowspiracy. The film follows […]

Price: (as of – Details) What the Health is the groundbreaking follow-up film from the creators of the award winning documentary Cowspiracy. The film follows […]

Product mailing list: http://signup.feelconfident.com @business https://youtu.be/1Yf3tYYNM-U Dr. Gary Linkov on Social Media: Instagram: https://www.instagram.com/drgarynyc Facebook: https://www.facebook.com/cityfp Twitter: https://twitter.com/drgarynyc City Facial Plastics: Dr. Gary Linkov 635 […]

Price: (as of – Details) From the brand Not just a brand Fashion • Fitness • Lifestyle Be active with us! Meet Your Inner Power […]

Price: (as of – Details) Product Description Peptide & Ceramide Blend A powerful peptide blend that supports skin’s natural repair mechanisms for younger, smoother looking […]

Price: (as of – Details) To calculate the overall star rating and percentage breakdown by star, we don’t use a simple average. Instead, our system […]

Scarlet Laser Treatment uses a high-quality radio frequency (RF) machine with fractional micro-needles. It delivers heat energy to the dermis directly to stimulate the production […]

Price: (as of – Details) JISLA Date First Available : March 13, 2023 Manufacturer : HAMGDYON ASIN : B0BY8G2QQ8 […]
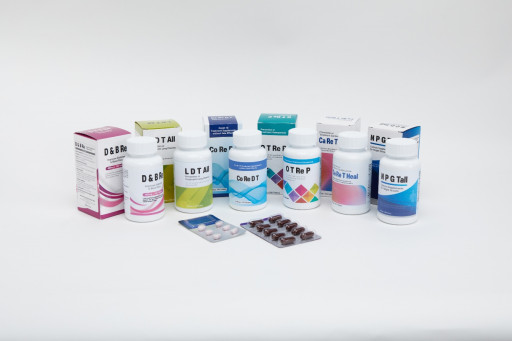

 Korean health bio product company QN Science introduces its Calcium supplements in the US market with its unique quantum natural nano manufacturing technology.
Korean health bio product company QN Science introduces its Calcium supplements in the US market with its unique quantum natural nano manufacturing technology.

SEOUL, South Korea, April 14, 2023 (Newswire.com)
–
Korean health product company QN Science announced on the 5th that it is entering the US market with its strict production standards for calcium products. QN Science’s dry nanotechnology, also known as the quantum natural nano manufacturing technology, can increase the absorption rate of calcium supplements and help reduce the risk of osteoporosis and maintain normal immune function.
This is a calcium product that is certified with ISO 9001, ISO 14001, and ISO 45001 and registered with U.S. FDA (Food and Drug Administration) for food and OTC. It shows enhanced digestion and absorption with homogeneous spherical nano-particles that are one hundred thousandths the size of a hair, which can help increase calcium absorption and bone density.
In addition to various global certifications for production and quality control, food safety management, and environmental management, the company has also registered with U.S. FDA and completed nutrition and safety tests at testing agencies.
The ISO 9001 certification is an international standard for quality management systems that verifies that the entire process of delivering services to consumers meets international standards. QN Science obtained this certification through efforts such as objectively responding to the diverse demands of internal and external stakeholders and systematically managing business procedures according to the manual.
The company also obtained ISO 14001, which is an environmental management certification for organizations and companies that establish environmental management as their business policy and continue to improve and maintain their systems.
Furthermore, as environmental concerns had become more important globally, QN Science has obtained ISO 14001 certification. It is a certification given to organizations and companies that establish environmental management as a business management policy and continuously carry out environmental improvements and system maintenance.
In addition, ISO 45001 is an international standard specification established by the International Organization for Standardization (ISO) for the Food Safety Management System, which aims to effectively manage potential hazards that may occur at each stage of food production, manufacturing, processing, preservation, and distribution through various certifications to challenge in the global market.
These products are named CO RE D T, CO DE R T, O T RE P, O RE CA P, D N B RE, D B TRE, L D T ALL, L B T A, CA RE T HEAL, and N P G TALL which can be met through the official website http://www.qnsci.com.
Seo Il-woo, CEO of QN Science, has announced, “The company has been using its unique quantum natural nanotechnology to research and develop Calcium supplements that can help restore the normal immune system in people who suffer from osteoporosis, cardiovascular disease, lung disease, diabetes, growth problems, and weakened immune systems due to COVID-19. The supplements are made with natural ingredients that are safe and free from side effects.”
He added, “Currently, QN Science has released 12 products, including six pharmaceuticals and six supplements made with the company’s unique quantum natural nanotechnology. The supplements are designed to maximize bioavailability and preserve active ingredients without damaging the natural properties of the ingredients. Our quantum natural nanotechnology is unique in that it allows to produce supplements with the highest level of safety and efficacy. QN Science plans to continue to release various versions of its Calcium supplements in the future.”
Quantum Mechanochemistry: Molecules under External Forces | Q-Chem
Contact Information:
Laura Choi
PR Manager
+82-2-518-0199
Original Source:
QN Science Challenges the U.S. Market With Globally Certified Dry Natural Nano Calcium


 Research shows that platelet-rich plasma (PRP) could help boost egg quality and quantity and improve the effectiveness of fertility treatments like in-vitro fertilization (IVF)
Research shows that platelet-rich plasma (PRP) could help boost egg quality and quantity and improve the effectiveness of fertility treatments like in-vitro fertilization (IVF)
…


 Curiteva pioneered and received clearance of the world’s first 3D printed, fully interconnected porous PEEK structure, utilizing internally developed Fused Filament Fabrication.
Curiteva pioneered and received clearance of the world’s first 3D printed, fully interconnected porous PEEK structure, utilizing internally developed Fused Filament Fabrication.
HUNTSVILLE, Ala., April 14, 2023 (Newswire.com)
–
Huntsville, AL based technology company, Curiteva announced a limited commercial release utilizing its recently FDA-cleared Inspire Porous PEEK HAFUSE Cervical Interbody System. Curiteva pioneered and received clearance of the world’s first 3D printed, fully interconnected porous PEEK structure, utilizing internally developed Fused Filament Fabrication.
The Inspire platform is manufactured utilizing the Evonik VESTAKEEP® i4 3DF PEEK high-performance polymer on a proprietary, patented 3D printer designed, programmed, and built by Curiteva.
Alex Vaccaro, MD, PhD, president of Philadelphia-based Rothman Orthopedic Institute shared, “I believe structure drives biology and the lattice PEEK architecture enabled by Curiteva’s 3D printing process represents an exciting advancement in spine, orthopedics, and neurosurgical procedures which involve any type of biologic implant.”
Kevin Foley, MD, Chairman of Semmes-Murphey Neurologic and Spine Institute and professor of neurosurgery, orthopedic surgery and biomedical engineering at the University of Tennessee Health Science Center stated, “The Inspire porous PEEK technology checks all of the boxes for an ideal interbody implant: fully interconnected porosity, modulus of elasticity equivalent to cancellous bone, strong biomechanical properties, radiolucency, and a bioactive surface for osseointegration.”
Randy Dryer, MD, Central Texas Spine Institute commented, “Interconnected porosity, pore size distribution, and nano-surface architecture are typically hallmarks of the most effective synthetic allografts. I believe this novel implant enhanced with HAFUSE nano-surface topography incorporates those features and presents an optimal environment for osteoprogenitor cells to move throughout the implant enhancing bone healing (fusion) and reducing risk of subsidence. I’m excited to offer this to my patients.”
“This new technology further demonstrates Curiteva’s commitment to developing and investing in disruptive technologies,” said Todd Reith, Inventor and Vice President of Emerging Technology. “Our unique architecture is the result of years of research and development in 3D printing. As this technology matures, with our internal expertise, we have already contemplated other applications outside of spine and orthopedics.” The company plans a full commercial launch in the United States later this year.
About Curiteva:
Curiteva is a privately held technology and manufacturing company dedicated to advancing spine surgery and improving clinical outcomes by partnering with providers and suppliers to deliver innovative and intuitive implant systems to the market. Our business is founded on a commitment to building world-class manufacturing, accelerating research and development, maintaining lean operational discipline, and delivering novel technology to meet the evolving needs of our customers and the patients they serve. For more information, please visit www.curiteva.com
Contact Information:
Kristen Kyzer
Director of Business Development
256.213.1057
Original Source:
Curiteva Announces First Surgeries Performed With Novel 3D Printed Porous HA PEEK Technology
Copyright © 2026 | WordPress Theme by MH Themes