Dr. Andrea Small-Howard discusses the company’s novel process for developing disease-specific, cannabinoid-based therapeutics.
Dr. Andrea Small-Howard discusses the company’s novel process for developing disease-specific, cannabinoid-based therapeutics.
LAS VEGAS – October 13, 2022 – (Newswire.com)
Dr. Andrea Small-Howard, President and Chief Science Officer of Gb Sciences (OTCQB:GBLX), a leading plant-inspired, biopharmaceutical research and drug development company, is demonstrating the potential efficacy of their proprietary cannabis-inspired mixtures today at the Canna Pharma 2022 Conference in San Diego, California. Dr. Small-Howard explains Gb Sciences’ novel process for developing disease-specific, cannabinoid therapeutics in her presentation entitled “Case Studies: Identification of Minimum Essential Therapeutic Mixtures from Cannabis Plant Extracts by Screening in Cell and Animal Models.” Gb Sciences’ case studies include scientific evidence demonstrating the potential efficacy of their cannabinoid-based therapeutic mixtures designed to treat Parkinson’s disease, chronic pain, or inflammation using their novel minimal essential mixture approach.
“Medicinal cannabis has shown great promise for the symptomatic treatment of many serious human disorders, but concerns around safety, consistency, legality, psychoactivity, and non-standardized routes of delivery, such as smoking and vaping, have slowed the adoption of the use of medical cannabis within traditional, physician-guided patient treatment programs. To address these challenges, we hypothesized that within Cannabis plant extracts, there would be a minimal essential mixture of compounds that preserves whole plant benefits, but with the manufacturing, quality control, and regulatory benefits of single ingredient therapies,” said Dr. Andrea Small-Howard.
Minimal essential mixtures (MEM) is a term Gb Sciences has coined from their proprietary research after deeming that a simplified mixture represented a happy medium between whole plant medicines and single ingredient drugs. Minimum Essential Mixtures are designed to retain increased therapeutic effectiveness from molecular synergies within the original plant extracts, but with the manufacturing efficiencies, quality assurances, and regulatory advantages of single ingredient drugs. Gb Sciences’ disease-specific MEM are produced for clinical evaluation using synthetic homologs of cannabis-based ingredients incorporated into sophisticated oral delivery modalities including Oral Dissolving Tablets (ODT), Oral Thin Films (OTF), time-released oral nanoparticles, and gel capsules to increase the stability, bioavailability, and ease-of-use of these ingredients relative to the non-standardized delivery methods used for Cannabis plant extracts.
Gb Sciences was recently published in Pharma’s Almanac discussing the strategy behind their plant-inspired minimum essential mixtures, which provides context for the case studies being presented today.
To learn more about Gb Sciences, visit www.gbsciences.com.
About Gb Sciences and GbS Global Biopharma
Gb Sciences, Inc. is a plant-inspired, biopharmaceutical research and development company creating patented, disease-targeted formulations of cannabis- and other plant-inspired therapeutic mixtures for the prescription drug market through its Canadian subsidiary, GbS Global Biopharma, Inc. The ‘plant-inspired’ active ingredients in its therapeutic mixtures are synthetic homologues identical to the original plant compounds but produced under current Good Manufacturing Practices. Gb Sciences’ intellectual property portfolio contains six issued U.S. and three issued foreign patents, as well as 17 U.S. and 51 foreign patent-pending applications. In its drug development pipeline, Gb Sciences has five preclinical phase product development programs. Gb Sciences’ lead program for Parkinson’s disease is being prepared for a first-in-human clinical trial. Gb Sciences’ formulations for chronic pain, anxiety, and depression are currently in preclinical animal studies with researchers at the National Research Council Canada. The company also received positive preclinical proof-of-concept data supporting its complex mixtures for the treatment of Cytokine Release Syndrome and its lead candidates will be optimized based on late-stage preclinical studies at Michigan State University. Gb Sciences’ productive research and development network includes distinguished universities, hospitals, and Contract Research Organizations. To learn more, visit www.gbsciences.com.
Forward-Looking Statements
This press release may contain statements relating to future results or events, which are forward-looking statements. Words such as “expects”, “intends”, “plans”, “may”, “could”, “should”, “anticipates”, “likely”, “believes” and words of similar import may identify forward-looking statements. These statements are not historical facts, but instead represent only the Company’s belief regarding future events, many of which, by their nature, are inherently uncertain and outside of the Company’s control. It is possible that the Company’s actual results and financial condition may differ, possibly materially, from the anticipated results and financial condition indicated in these forward-looking statements. Further, information concerning the Company and its business, including factors that potentially could materially affect the Company’s business and financial and other results, are contained in the Company’s filings with the Securities and Exchange Commission, available at www.sec.gov. All forward-looking statements included in this press release are made only as of the date of this press release, and we do not undertake any obligation to publicly update or correct any forward-looking statements to reflect events or circumstances that subsequently occur or of which we hereafter become aware.
Contact Information:
Madeleine Moench
[email protected]
Press Release Service
by
Newswire.com
Original Source:
TODAY: Gb Sciences’ President Demonstrates Effectiveness of Simplified Therapeutic Mixtures Inspired by Cannabis Plant Extracts at Canna Pharma 2022 Conference

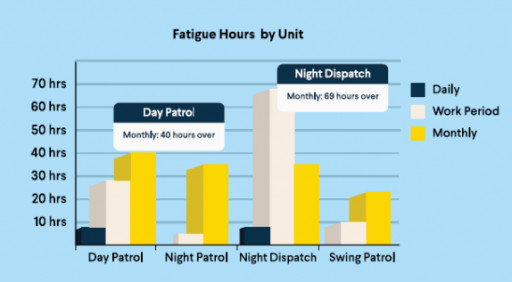

 A data-driven wellness solution with fatigue monitoring capabilities using scheduling insights enables law enforcement agencies to take a proactive approach to officer health and well-being.
A data-driven wellness solution with fatigue monitoring capabilities using scheduling insights enables law enforcement agencies to take a proactive approach to officer health and well-being.