VidaTalk’s Talk-to-Translate Feature Will Have a Transformative Impact on Hospitalized Patients With Language Barriers

 Achieving the standard of care, no matter the language or disability.
Achieving the standard of care, no matter the language or disability.
ANN ARBOR, Mich., July 19, 2023 (Newswire.com)
–
Vidatak is proud to announce the latest feature, Talk-to-Translate, has just been added to VidaTalk to better ensure providers can deliver equitable care to patients with communication barriers and disabilities on a 24/7 basis.
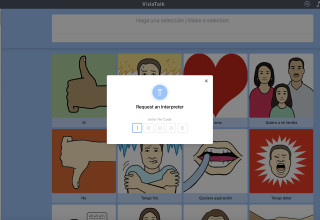
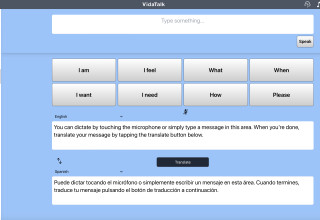
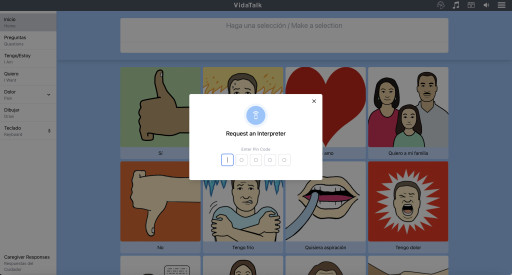
VidaTalk is a first-in-kind, multilingual, point-of-care communication solution for patients with speech, hearing and language barriers. This customizable app for patients and providers includes picture-option communication selections that generate bilingual text and audio output when selections are made across 40 language profiles, relaxation videos, therapeutic restorative music, video and audio interpreter access, talk-to-text, and now, talk-to-translate. The new talk-to-translate feature released today ensures that even under the rarest circumstances, patients and providers will be able to understand one another.
Language barriers pose significant challenges in healthcare where effective and timely communication is vital. While in-person and remote video and telephonic interpreter services provide this essential service as the standard of care, they simply are not always feasible or available with every patient encounter, especially for languages spoken with lower frequency.
With delayed or absent access to interpreters, providers find themselves unable to communicate with or understand their patients. This communication gap exposes patients to significant risk of harm, misdiagnosis, delayed diagnosis, and delayed or absent treatment. While for complex conversations, such as discharge instructions, Google Translate has been shown to be unreliable, in certain circumstances, it may be necessary.
“As VidaTalk continues to harness the technologies that drive equitable experiences for vulnerable patient populations, it is essential for hospitals to consider what their standard of care actually delivers with respect to a real-time multilingual experience and a human-centered approach to care.” – Dr. Patak, CEO of Vidatak
Vidatak’s Chief Experience Officer, Lisa Spencer, shares a personal experience she had with a loved one: “My family member could not communicate his needs and symptoms. It was frustrating and heartbreaking for everyone. I’m not alone. Every hospital is caring for patients with speech, hearing and language barriers. For patients that don’t speak the same language as their caregivers, using certified interpreters is the gold standard; but, when no one with language services is available, patients still need to communicate. That’s why I’m so excited that VidaTalk now has this new talk-to-translate feature. No one deserves to suffer or be in pain for hours, or even an entire weekend, simply because an interpreter can’t be reached.”
By ensuring that multilingual communication can occur in real time at the point of care, VidaTalk validates the highest standard of care, establishing a mechanism for effective communication for patients who have speech, hearing or language barriers. This vital connection can alleviate patient distress, enhance trust, and contribute to a more positive healthcare experience. When patients feel understood and supported, they are more likely to actively engage in their own healthcare journey, leading to better compliance, faster recovery, and enhanced overall well-being.
VidaTalk has revolutionized the healthcare experience for hospitalized patients who cannot speak, cannot hear, or have limited English proficiency. “As VidaTalk continues to harness the technologies that drive equitable experiences for vulnerable patient populations, it is essential for hospitals to consider what their standard of care actually delivers with respect to a real-time multilingual experience and a human-centered approach to care,” states Dr. Patak, CEO of Vidatak.
Contact Information:
Lance Patak
CEO
(323) 697-9906
Lisa Spencer
Chief Experience Officer
(425) 247-4007
Related Files
Adults with Communication Disabilities Experience Poorer Health and Healthcare Outcomes Compared to Persons Without Communicatio
Giving Voice- Nurse-Patient Communication in the Intensive Care Unit.pdf
Related Images
Original Source:
VidaTalk’s Talk-to-Translate Feature Will Have a Transformative Impact on Hospitalized Patients With Language Barriers
The post VidaTalk’s Talk-to-Translate Feature Will Have a Transformative Impact on Hospitalized Patients With Language Barriers appeared first on AESTHETIC NEWS.